
Streamlining approval workflows, meeting regulatory standards and simplifying audit traceability

The University of Basel’s Department of Clinical Research required secure and compliant document review.
Approvals for Confluence streamlined their workflows, ensured traceable sign-offs, and future-proofed the vitally important work they produce.
A great app with a very responsive team. A key part of our regulatory documentation approval processes.
At a glance
Operating in a highly regulated academic and clinical environment, The Department of Clinical Research at the University of Basel plays a vital role in advancing medical science through rigorous research.
But to strengthen compliance, the department’s IT services team needed a solution to introduce structured, auditable approval processes directly into the department’s existing Confluence pages.
The answer?
Discover how the team gained a lightweight but powerful way to ensure key documents were signed off and compliant with internal and external regulatory standards, without introducing unwieldy third-party tools.
The
Customer
Name University of Basel, Department of Clinical Research, IT Services team
Industry Medicine / Clinical Research
Founded 2007
HQ Switzerland
Use case Supporting the digital infrastructure that underpins vital research efforts, managing essential documentation for Computer System Validation
The Challenge
Ensuring proper review and approval for all validation documentation
Meeting rigorous regulatory standards for documentation management
Retaining approval history within Confluence itself, independent of external apps
With documentation central to their compliance processes, the IT team needed a secure, structured way to approve critical documents related to computer system validation.
In the absence of a simple built-in way to manage and persist document approvals within Confluence itself, the team needed a solution that offered reliable, long-term traceability, without compromising usability or requiring excessive human intervention.
Did you know?
The Solution
The IT team at the Department of Clinical Research implemented Approvals for Confluence to streamline their document review process.
The ability to persist approval history as Confluence-native comments provided the team with the auditability they required, and strengthened long-term resilience, ensuring critical metadata remained embedded in their documentation.
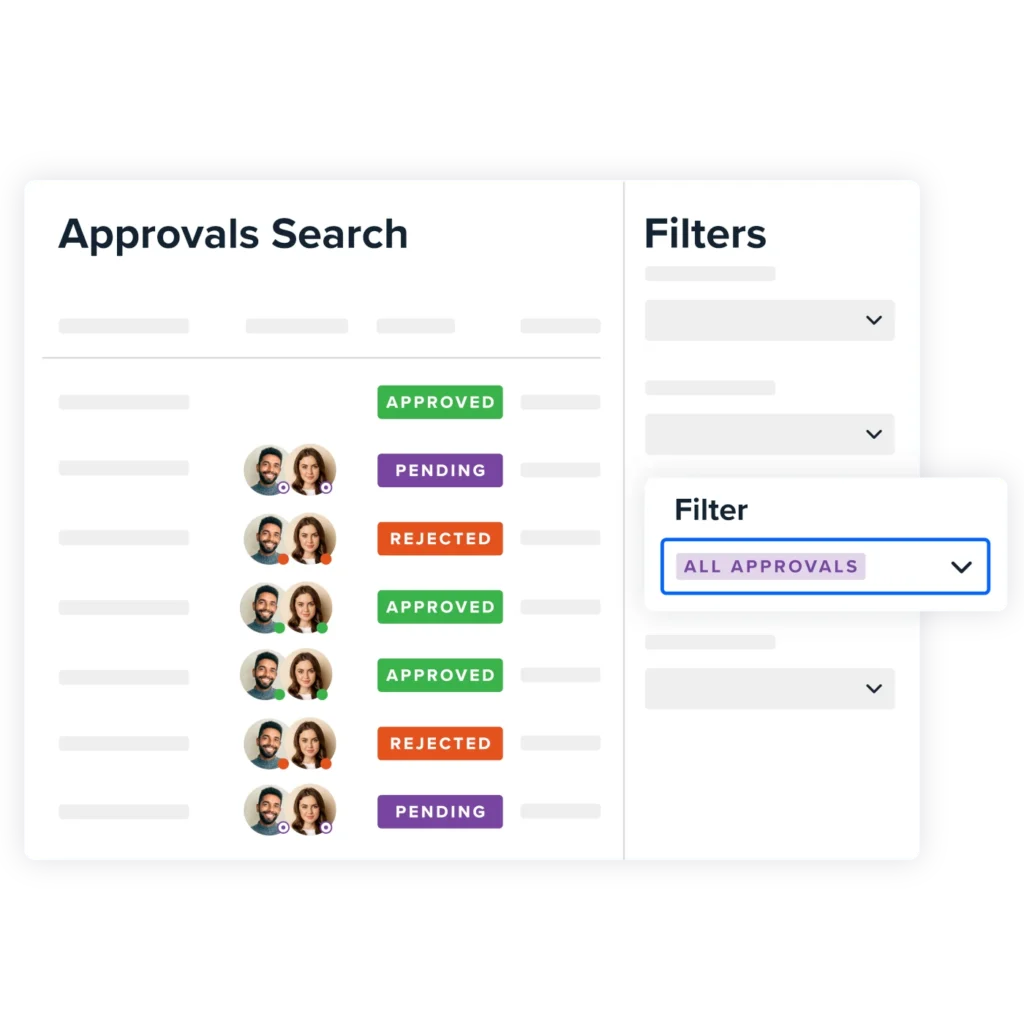
With Approvals for Confluence, the team could:
Assign approvers to key documents with ease
Record approvals visibly and transparently
Meet compliance requirements without relying on email chains or external systems
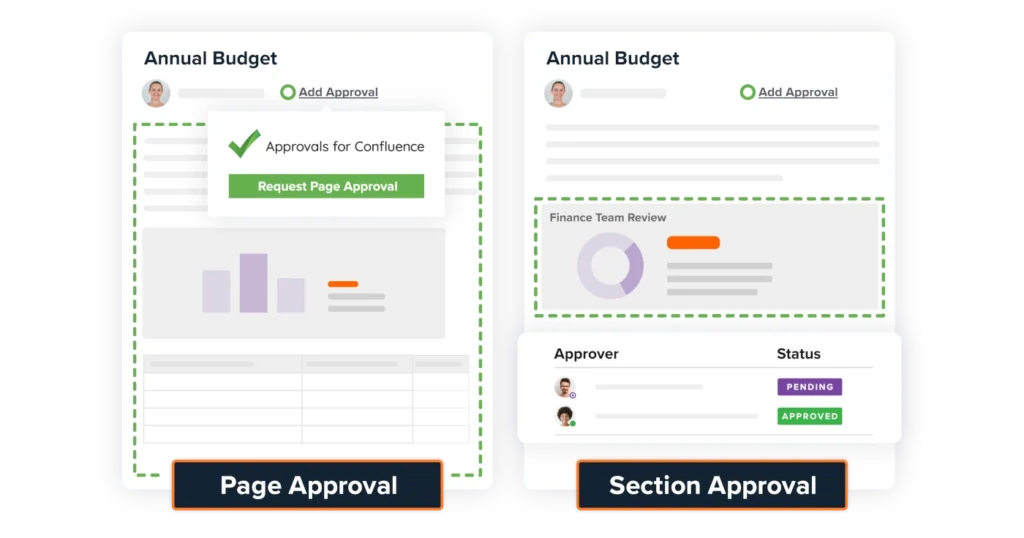
The Results
Approvals for Confluence is now a central component of the department’s documentation strategy, supporting the rigorous demands of both the academic environment and the clinical research and med-tech sectors.
By adopting Approvals for Confluence, teams like this one are able to achieve:
*statistics produced by AIIM and Forrester
Try it today
Approvals
for Confluence
Ensure quality. Simplify approvals. Work where your content already lives.
Discover for yourself how Approvals for Confluence can help your team today.



